One of the more recent additions to the global COVID-19 vaccine roster comes from Novavax, whose vaccine is poised to hit worldwide markets and hopefully make a big difference in the big problem of global vaccine equity. 🌏
Novavax’s shot uses a tried-and-true technology: it’s called a protein subunit or acellular vaccine. It’s the same tech used to make the vaccines already available to protect us from pertussis, pneumonia, and hepatitis B. Protein subunit vaccines have several advantages, including that they are well-tolerated, relatively cheap, relatively low-tech to manufacture, and don’t need to remain frozen.
Novavax is one of several pharmaceutical companies worldwide working on a vaccine using a protein subunit. Many are going to be hitting markets soon, and a few already have. Taiwan has been locally producing and distributing one called Medigen since August, and Novavax is already available under an emergency use authorization in Indonesia and the Philippines.
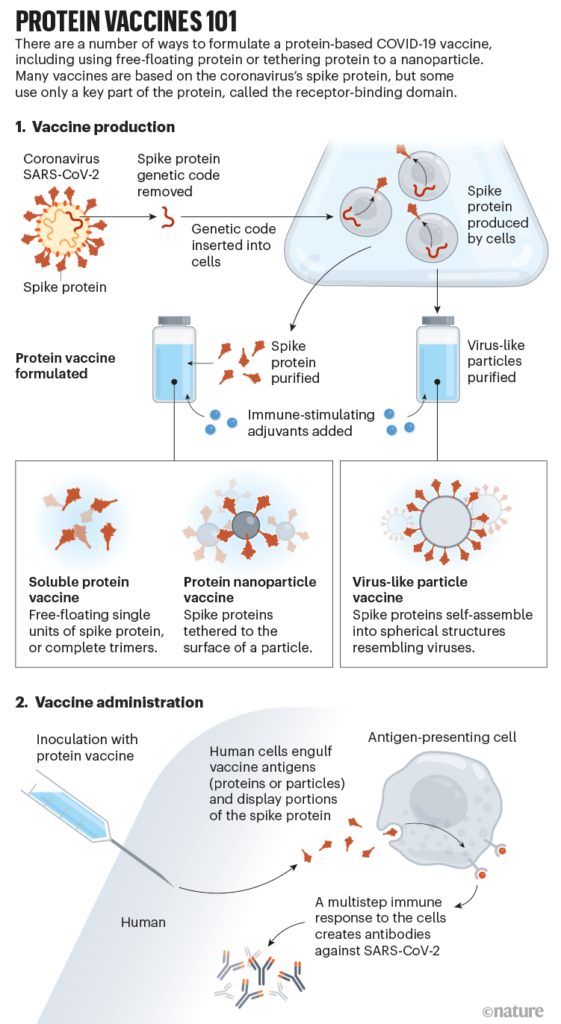
Here’s how it’s done, in brief: the genetic code for only the virus’ spike protein (or only part of it) is extracted and inserted into a cell line. The DNA-edited cells are cultured and they make the spike protein. The proteins are extracted from the cell culture and purified, then manufactured into a vaccine. We’re borrowing a very helpful illustration of these steps from the Nature article linked below.
Novavax uses a moth cell line to do this (yes, the fuzzy flying insect). Just like other cultures, we’re talking about a bunch of identical cells in a dish 🧫–not a mutant moth. 🦋
Protein subunit vaccine development is on a slower timeline than mRNA and viral vector vaccines because it involves many steps which must each be perfected before moving on–a slow process.
Novavax was hoping for a slightly speedier release of its product but hit a series of unwelcome delays in testing and manufacturing. Sanofi’s protein subunit vaccine similarly hit manufacturing hurdles early on.
Results of the Phase III clinical trial of the Novavax shot had on average 90% efficacy for preventing symptomatic COVID, and as much as 100% efficacy for moderate or severe disease. (Efficacy was somewhat sensitive to the variant in circulation when the trial was conducted, so there are several different efficacy estimates. The *lowest* was 86.3% against symptomatic illness when Delta was in circulation.)
It also has a very good safety profile and fewer annoying side effects than mRNA vaccines.
We don’t have any data yet on how well Novavax works against the Omicron variant, but they are testing now–and they have said they could start producing an Omicron-specific shot in January.
The vaccine from Novavax received its first emergency use approvals in Indonesia and the Philippines in November 2021. They have filed for authorization in many other countries too–including the UK, Australia, New Zealand, Canada, South Korea, India, the United States, and the EU.
It’s exciting news because of the logistical advantages of these vaccines. They’re cheap and (relatively speaking) low-tech to produce and can be shipped unfrozen. As of December 1, 2021, just 8% of people in low-income countries worldwide have had at least 1 dose, compared to 65% of people in high-income countries. The emergence of the Omicron variant in South Africa, where just a fraction of people are fully vaccinated, illustrates the absolute necessity of global cooperation to achieve vaccine equity and an end to the pandemic.
Sources:
How protein-based COVID vaccines could change the pandemic
All Updates On Our COVID-19 Vaccine Effort
EU nears decision on Novavax COVID-19 vaccine
Global Dashboard for Vaccine Equity
Image credit: Nik Spencer/Nature


