💥BREAKING💥: 3rd dose COVID-19 vaccine authorization & recommendation for immunocompromised in the U.S.
Yesterday (August 12th,) the U.S. Food and Drug Administration (FDA) authorized an additional dose of the Pfizer and Moderna vaccines for certain immunocompromised individuals ages 12+.
Today (August 13th) the CDC’s Advisory Committee on Immunization Practices (ACIP) also voted to recommend these additional doses.
💥 This means that additional doses can be given immediately.
This recommendation is based on evidence that certain immunocompromised individuals do not mount as effective of an immune response to the vaccines, but a 3rd dose can help push many (but not all) of these low responders above the threshold of protection. Immunosuppressed individuals are also more susceptible to severe COVID-19 complications.
Key take-aways from the FDA and ACIP:
The CDC’s definition of “moderately and severely immunocompromised people” for whom 3rd doses are recommended includes:
➡️ Active treatment for solid tumor and hematologic malignancies
➡️ Receipt of solid-organ transplant and taking immunosuppressive therapy
➡️ Receipt of CAR-T-cell or hematopoietic stem cell transplant (within 2 years of transplantation or taking immunosuppression therapy)
➡️ Moderate or severe primary immunodeficiency (e.g., DiGeorge, Wiskott-Aldrich syndromes)
➡️ Advanced or untreated HIV infection
➡️ Active treatment with high-dose corticosteroids (i.e., ≥20mg prednisone or equivalent per day), alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents classified as severely immunosuppressive, TNF blockers, and other biologic agents that are immunosuppressive or immunomodulatory
❇️ The 3rd dose should be given at least 28 days after the 2nd dose.
❇️ Whenever possible, mRNA COVID-19 vaccination doses (including the primary series and an additional dose) should be given at least two weeks before initiation of immunosuppressive therapies.
❇️ The additional dose should match the mRNA primary series (Pfizer or Moderna), but if that is not feasible, a different additional dose is allowed.
❇️ Testing to assess immune response to vaccination and need for an additional dose is not established and NOT recommended.
While the committee says the people with more common chronic illnesses such as diabetes are not meant to be included in this guidance, the above list is also not exhaustive and gives discretion to physicians and patients. (Please don’t ask us about your specific situation, talk to your own doctor!).
While the new U.S. recommendation is welcome news for the 2.7% of the U.S. population estimated to be immunosuppressed, it didn’t answer ALL the burning questions.
For example, the FDA said they were unable to extend the authorization for an additional dose to the Johnson & Johnson/Janssen vaccine due to insufficient data (though they re-iterated they hope to have data on this soon). We know this is frustrating to many (including us).
The committee will also continue to evaluate the need for boosters for a broader subset of the population.
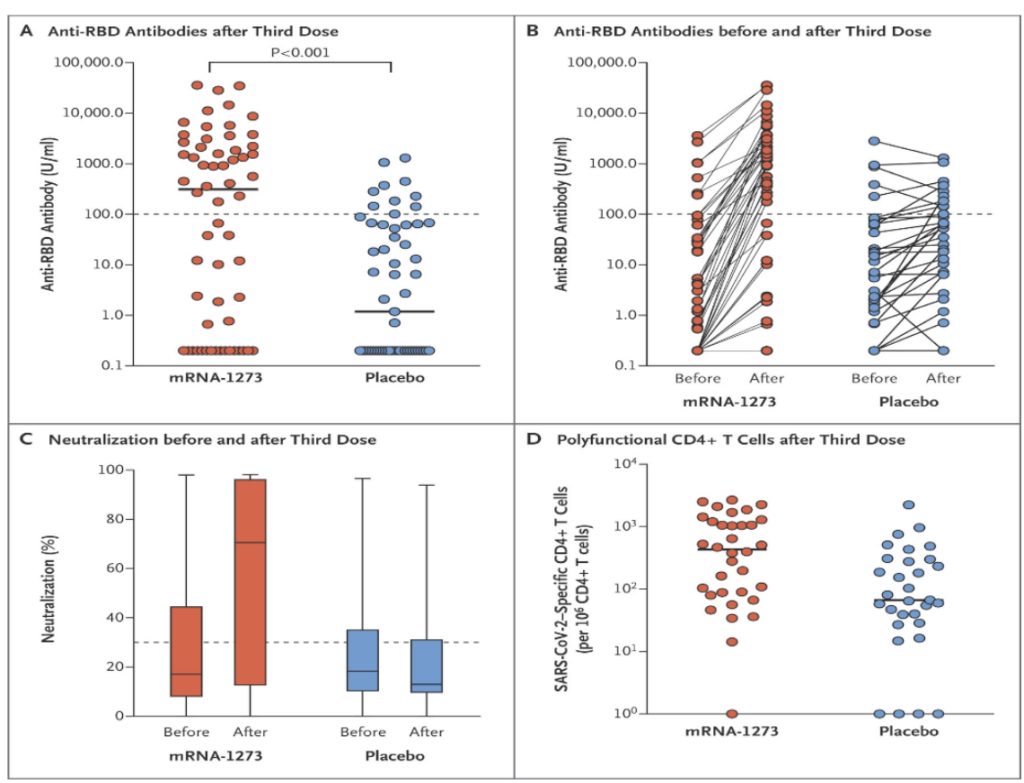
⚠️ Finally, while a 3rd dose has been shown to increase immune response among the severely immunocompromised, there is still a sizeable proportion of this group who still did not mount detectable antibodies with a 3rd dose.
In studies of transplant recipients or patients on hemodialysis, among those who had no detectable antibody response to an initial mRNA vaccine series, 33-50% developed an antibody response to an additional dose. This is great but shows that some are still left with low protection even after a 3rd dose.
Given this remaining risk coupled with the current Delta surge, the CDC emphasized that immunocompromised people, including those who receive an additional dose, should continue to follow prevention measures for the time being.
This includes wearing a mask, staying 6 feet apart from others they don’t live with and avoiding crowds and poorly ventilated indoor spaces.
Close contacts of immunocompromised people should be strongly encouraged to be vaccinated against COVID-19.
➡️ BOTTOM LINE:
3rd doses now recommended for moderately to severe immunosuppressed in the U.S.
💥 ACIP said it should be left to the patients and doctors to decide who needs an extra dose and what the timing of that dose should be. People will be left on their own to attest to their need for the third dose — no prescription or doctor’s note will be needed.
Links:
Slides from the ACIP meeting with great summary of the current studies on vaccines and immunosuppression
Recent DP summary of vaccines and immunosuppression
Figure source:
Hall et al. (2021) NEJM. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients


